Abstract
Introduction: Therapy related myeloid neoplasm (t-MN) is a rare but devastating complication of cytotoxic therapy used for management of unrelated malignancy or autoimmune diseases. Therapeutic options for t-MN are limited and although hypomethylating agents (HMA) are frequently used, the benefit of it in t-MN is unclear. There are no prospective trials of HMA in t-MN and published experience is based on retrospective analysis of small number of cases (n=42 to 54). The aim of this study was to assess the efficacy of HMA therapy in t-MN patients and evaluate predictors for it.
Methods: t-MN patients managed with first line HMA therapy were analyzed. This study was approved by the participating institutional review board. Criteria for inclusion were a pathologically confirmed diagnosis of t-MN based on WHO 2016 criteria, age >18 years, and ≥1 cycle of decitabine or azacitidine.
Patient characteristics were summarized by frequency (percentage). Overall survival was calculated from the time of starting azacitidine to last follow up or death date using Kaplan-Meier analysis. Multivariable analysis was performed using Cox proportional hazard model. Statistical analysis was performed using R program and significance was defined as P<0.05.
Results: Of the 554 t-MN patients analyzed, 184 (33%) patients were treated with HMA as the first line therapy and were included in the study. Median age at t-MN diagnosis was 70 (IQR 64-75) years and 112 (60%) patients were male. At the time of diagnosis, 84% (n=154) and 16% (n=30) patients were classified as t-MDS and t-AML and 47% (87/184) and 46% (49/107) of the evaluable patients had complex karyotype and TP53 somatic mutations (TP53 Mut) respectively. Azacitidine (n=145, 79%) was most frequently used HMA followed by decitabine (n=39, 21%). Median survival of the whole cohort since t-MN diagnosis was 13.2 months.
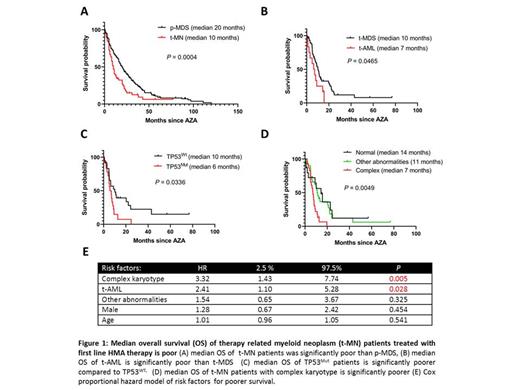
At the time of analysis further treatment details were available for 78 patients and outcome of these patients were compared with primary MDS (p-MDS) and oligoblastic AML managed at the same institute. Completion of six-cycles of HMA (50% vs. 62%; P=0.07) and overall response rate (47 vs. 50%, P=0.22) was not significantly different between t-MN and p-MDS. However, median OS of t-MN was significantly poor compared to p-MDS (10 vs. 20 months, P=0.0004; Figure 1A).
In t-MN patients treated with HMA as the first-line agent, median OS was significantly shorter in t-AML compared to t-MDS (7 vs.10 months, P=0.04; Figure 1B). Median OS was significantly shorter in patients with TP53 Mut and complex karyotype (Figure 1C-D). In Cox proportional hazard analysis, t-AML and complex karyotype were independently associated with poor outcome.
Conclusion: This study demonstrates significantly poor outcome of t-MN patients treated with HMA therapy as first line. The outcome is particularly poor in t-AML and patients with complex karyotype and TP53 Mut. Hence there is urgent need for clinical trials of novel therapies for t-MN.
Sharplin: Kite Gilead: Honoraria; Novartis: Other: Travel Support. Ross: Keros Therapeutics: Consultancy, Honoraria; Bristol Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal